The Strength of an Acid or Base Depends on
On which factor does the strength of an acid depend. The stronger the bond the max energy is required to break it.
The strength of an acid or base is called acidity.

. Values of the pH of 010 M solutions of a number of common acids and bases are given in the table below. Acidity is measured by pH which is the concentration of hydrogen ions in a solution. Strength of acid is as follows.
The pH of a solution depends on the strength of the acid or base in the solution. If the bond is very polar the proton tends to go away the molecule more easily making it a robust acid. In water so it is a strong acid but Acetic acid partially ionises in water so it is a weak acid.
As the pH diminishes the acidity is greater. It is proportional to its ionization and pH decreasement. Factors Affecting Acid Strength.
The strength of acids and bases depends on the number of H and OH ions produced. The strength of an acid is the amount of Hydrogen ions it forms when it ionises. It depends on how much of the substance breaks down into ions when it dissolves in water.
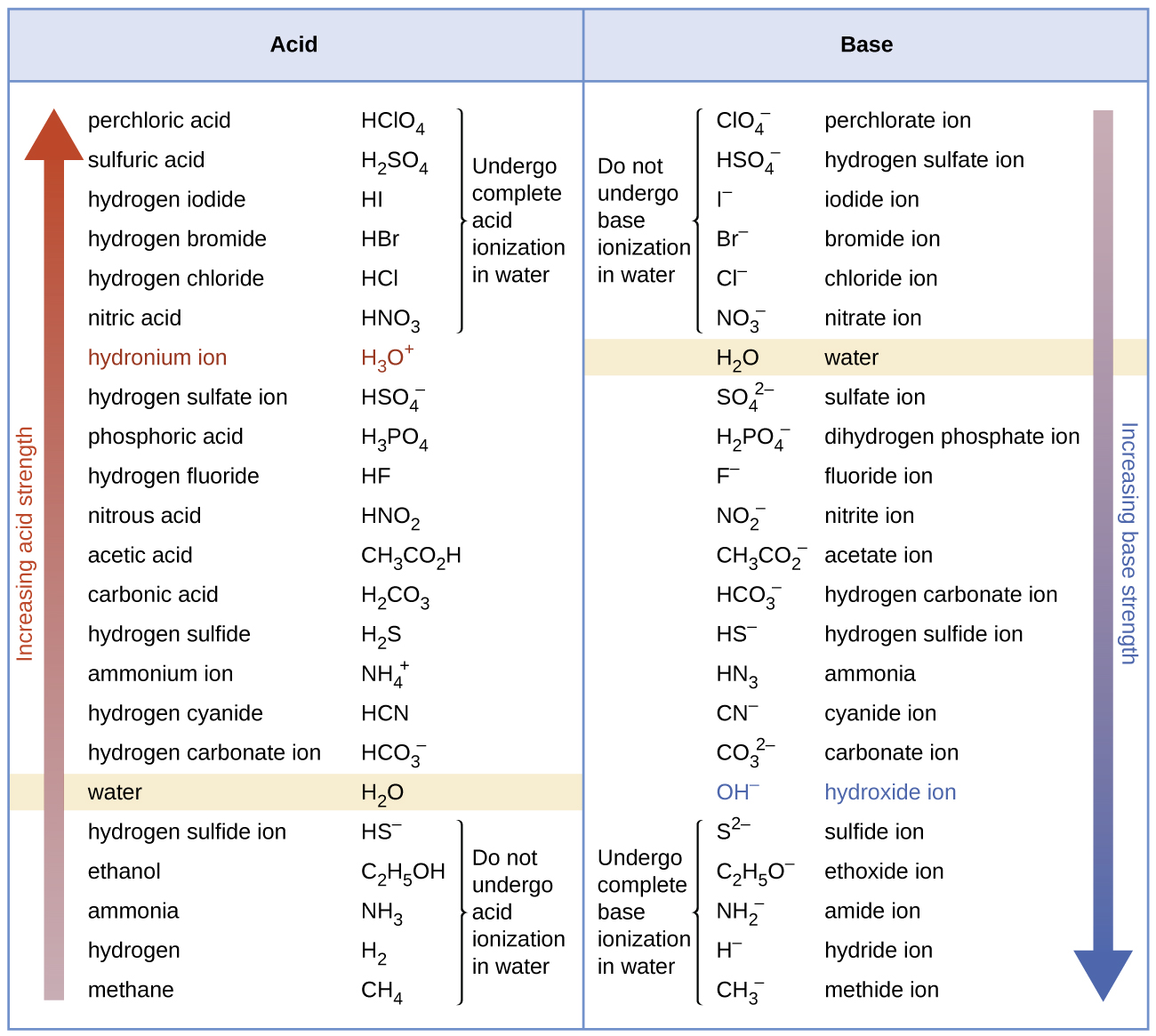
Acid base When a strong acid dissolves in water all none or a small fraction molecules ionize into ions. It depends on how much of the substance breaks down into ions when it dissolves in water. The strength of an acid depends on the concentration of hydronium ion H 3 O too.
What do you understand by the strength of an acid. The pH of the solution will depend on the relative strengths of the acid and base from which the salt is made. Chemistry questions and answers.
Acids that provide more number of H ions are known to be strong acids and vice versa. A strong acid will completely ionize in water while a weak acid will only partially ionize. A strong acid or base completely ionizes in solution.
It depends on the number of Hydrogen ions H and is directly proportional to it. The acid strength is affected by the polarity of the H-A bond. The graph represents a.
If the acid is stronger the solution will be acidic while if the base is stronger the solution will be basic. Measurements of the pH of dilute solutions are therefore good indicators of the relative strengths of acids and bases. The strength of an acid or base depends on how many _____ or _____ particles dissociate into ions in water.
How much more acidic is a solution with a pH of 4 than a solution with a pH of 7. Acid strength is mainly depend on the active hydrogen the H atoms attached with highly electronegative atoms such as FNO etc or with some groups like CO gp. The weaker the bond the lesser the energy required to break it.
An acid or bases strength refers to its degree of ionization. In the following reactions an acid or a base reacts with water to produce conjugates Strong acid is an effective proton donor that is assumed to completely dissociate in water. Acidity is an important factor for living things because most can survive only within a relatively narrow range of acidity.
Acid or base strength is a measure of how readily the molecule ionizes in water. Acids and bases behave differently in a solution based on their strength. A solution with a pH of 4 is 1000 10 10 10 or 103 times more acidic than a solution with a pH of 7.
If the bond is highly polar the proton tends to leave the molecule more easily making it a strong acid. A substance with pH acidic pH7 - basic. Acidity is measured by pH which is the concentration of hydrogen ions in a solution.
The strength of an acid depends on the H-A bond. When the pH increases the acidity is lesser. Buffer capacity will predominately depend on Select the correct answer below O the strength of the acid or base being added O the strength of the acid and base in the buffer O the concentration of acid or base being added O the concentration of the buffer solution MORE INSTR.
If it dissociates completely it is a strong acidbase. Which order of arrangement is correct in terms of the strength of the acid. The strength of acids and bases depend on how much an acid or base ionizes in solution.
Correct answer to the question The strength of an acid or base depends on the degree on of ionization in water. Thus the basicity increases. The strength of an acid depends on its tendency to______.
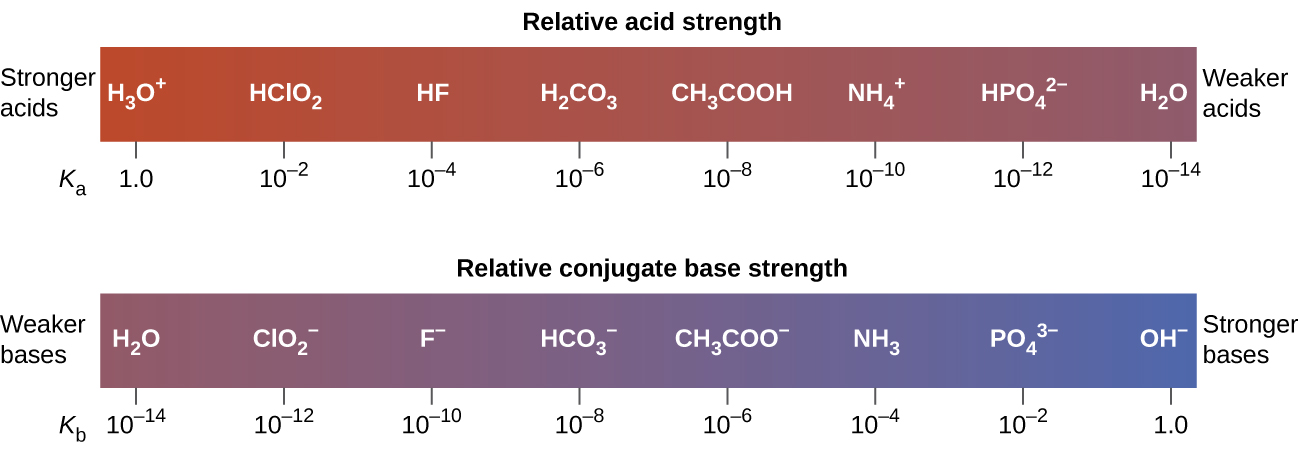
The strength of an acid in an organic compound is directly proportional to the stability of the acids conjugate baseOr an acid that has a more stable conjugate base will be more acidic than an acid that has a less stable conjugate baseAcidic molecules generally have structural features that allow the anion in the conjugate base to delocalize the charge over a larger space. It depends on the strength of the H-A bond. The strength of an acid or base can also be determined by the degree of ionisation of acids and bases which differ for.
The strength of an acid or a base depends on the degree to which it dissociates. Conjugate base of strong acid is weak base. Strength of an acid or base depends on the degree to which it ionizes dissociates in water.
The strength of an acid or a base depends on how much of it breaks down into ions when it dissolves in water. If the acid and base are of equal. The polarity of the H-A bond affects its acid strength.
Hence the acid is weak. For instance HCl fully ionises. Which of the following orders of relative strength of acid is correct.
Acidity is an important factor for living things because most can survive only within a relatively narrow range of acidity. The strength of an acid or base depends on the degree on of ionization in water. Hence the acid is strong.
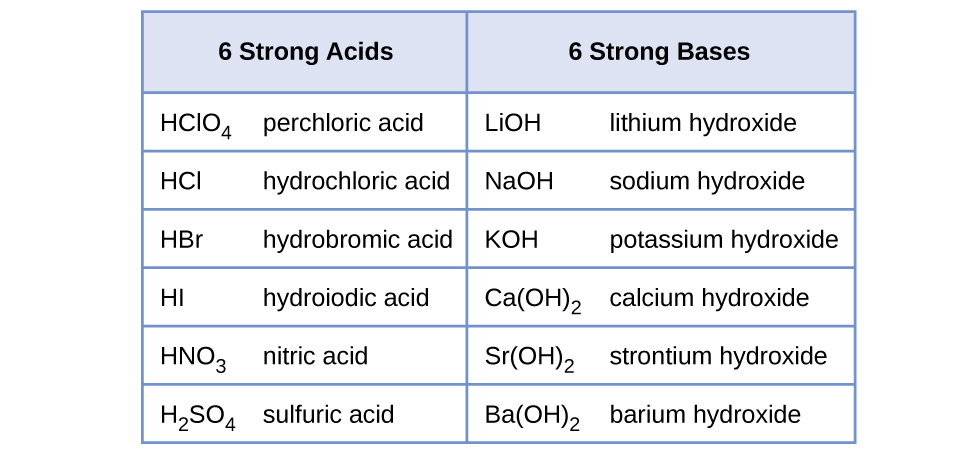
The strength of an acid or base is called acidity. HCl H 2 SO 4 and HNO 3. In a neutralization reaction an acid and a base react to produce a salt.
A salt is an ionic compound whose cation comes from a base and whose anion comes from an acid.

Feministstalk On Instagram Teach Your Daughters To Be Financially Independent And Self Sufficient Instead Daughter Quotes Independent Quotes Warrior Quotes

Strong Acids And Strong Bases Acids And Bases That Are Strong Electrolytes Completely Ionized Chemistry Education Teaching Chemistry Organic Chemistry Study
14 3 Relative Strengths Of Acids And Bases Chemistry

Thin Layer And Column Chromatography Source Https Www Chemguide Co Uk Analysis Chromatography Thinlay Thin Layer Chromatography Chemistry Organic Chemistry

Awesome All Mr Parr Science Songs Science Middle School Science Science Earth Science

Strength Of Acids And Bases How To Find It Chemistry Teachoo
14 3 Relative Strengths Of Acids And Bases Chemistry

Https Www Slideshare Net Educationchemist Acids And Bases 2230135 Dissociation Molecules Equations
14 3 Relative Strengths Of Acids And Bases Chemistry

Acids And Bases In Organic Chemistry Mcat And Organic Chemistry Study Guides Tutoring Organic Chemistry Chemistry Study Guide Organic Chemistry Study

Solvent Effects On Chemical Reaction Chemical Reactions Science Chemistry Chemical Science

Strength Of Acids And Bases Time Saving Science Video By Brightstorm Ap Us History Teaching American History History Videos

Pin On Acids Bases And Solutions

Strength Of Acids And Bases Concept Chemistry Video By Brightstorm

Pin By The Project Artist On Understanding Science In 2022 Science Understanding





Comments
Post a Comment